Limited time offer: $99 membership fee + included $20 side effect essentials kit

By 2030 nearly 3 billion adults will be overweight or obese worldwide.¹ eMed’s mission is to combat today’s obesity epidemic with responsible access to proven GLP-1 treatment and long term, holistic support.
We offer branded, FDA-approved GLP-1 medications paired with a structured program designed to be clinically sound, affordable, and sustainable.
1 World Obesity Atlas 2025.

In a market flooded with unregulated counterfeits and inconsistent sourcing, we remain committed to a higher standard.
Understanding where your health stands at the start allows us to measure change with accuracy and intention. Every patient begins with a blood test to establish a personal health profile. This is not meant as a barrier but as the foundation for responsible prescribing and long-term individualized progress tracking.

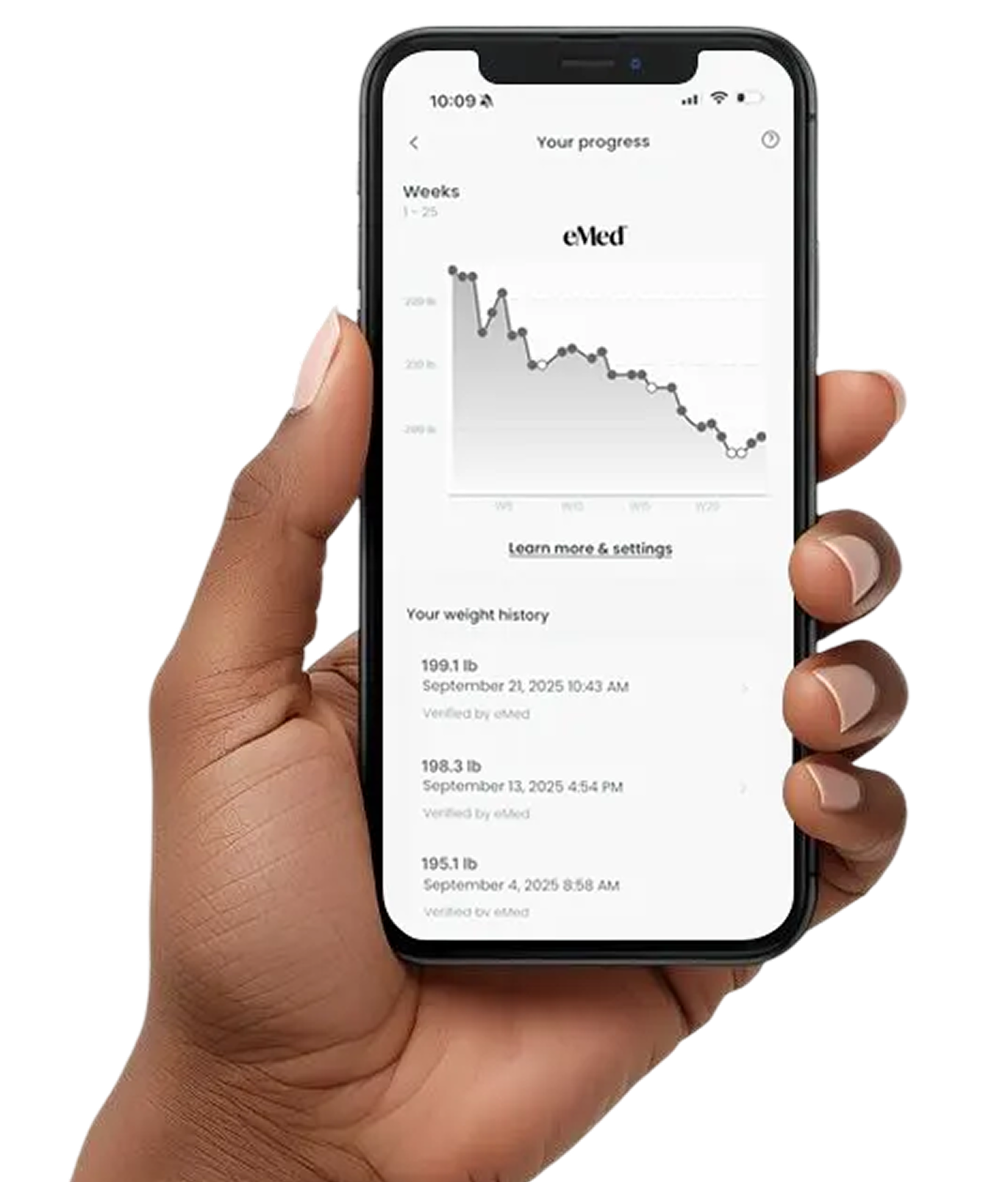
We believe the true differentiator in outcomes is adherence. GLP-1 medications generate clinically significant results when taken consistently over time.2 Our program is designed for real-life use with access to clinicians, digital health communication, and structured touch points that reduce friction and increase follow-through.
2 American Diabetes Association, Standards of Care in Diabetes 2024.
Semaglutide and tirzepatide medications mimic a natural peptide your body already produces, called GLP-1 (glucagon-like peptide-1). This peptide helps regulate appetite, manage blood sugar, and slow digestion.3
By activating these same pathways, medications like Wegovy® and Zepbound® help you feel full sooner, stay full longer, and eat less without feeling restricted.
Wegovy® important safety information
Zepbound® important safety information
Ozempic®* important safety information
3 NEJM, Semaglutide STEP 1 Trial, 2021.
* Ozempic has not been reviewed or approved by the FDA for weight-loss use. Any prescription decisions are made independently by licensed clinicians based on individual clinical evaluation.


Download the eMed app, and once your Screening Kit arrives, scan the QR code on the kit to begin the at-home lab collection.

A licensed clinician will review your medical profile and lab results.

If eligible, your GLP-1 prescription will be sent to your door.

Track your progress with a quick 60-second weekly check-in through the app, supported by 24/7 side-effect guidance and monthly clinician reviews.